Compiled by Brooke Warres and Phil Brannen, University of Georgia Plant Pathology Department
Due to a warm, humid climate that promotes multiple diseases, growing grapes in Georgia and the Southeast as a whole is a difficult task. Fortunately, with the right information, growers have been able to control harmful pathogens and expand the Georgia wine industry. In 2017, the value of Georgia’s wine grape industry was estimated at $18 million, but this does not include the amount of money brought in through agritourism. Vineyards are also popular event venues, bringing in people from all over the Southeast. To keep vineyards beautiful and productive, fungicides are essential in the fight against disease. Growers begin spraying their vines at budbreak and may continue spraying well past harvest to prevent losses for the following year, with some growers spraying over fifteen times. While grape spray program development can be somewhat overwhelming, there are many different registered fungicides available – unless pathogens develop fungicide resistance.

Sarah Campbell, a previous graduate student at University of Georgia, conducted a survey of downy mildew (Plasmopara viticola) to determine if resistance was developing in the quinone outside inhibitors (QoIs [FRAC 11]; e.g. Abound), a very popular and often-utilized chemical class. After testing isolates from all over the state, Sarah was able to conclude that QoI resistance in downy mildew is prolific in Georgia, thus largely rendering these chemicals useless in the fight against downy mildew. QoIs, however, are registered to control other pathogens of grape, so should they still be included in spray programs?
Next to downy mildew, powdery mildew (Erysiphe necator) is one of the most difficult to control diseases of grape in Georgia. While powdery mildew is similar in name to downy mildew, it is very different in its biology. Downy mildew, while appearing fungal like, is actually an oomycete (a group of organisms more closely related to brown algae than fungi) that thrives in warm, humid conditions; it needs water to spread. Powdery mildew is a true fungus that flourishes during drier summers; unlike most fungi, this pathogen does not require water for infection, and it actually develops and spreads readily in drier climates. Powdery mildew can grow on all green tissues where it exhibits “powdery” white growth (Fig. 1) on leaves, fruit, and canes. If not controlled, powdery mildew can reproduce multiple times through the growing season and quickly cause a severe epidemic. Even limited infection by this pathogen can produce off flavors in wine, making this disease a top priority for all wine grape growers in every region of the world.
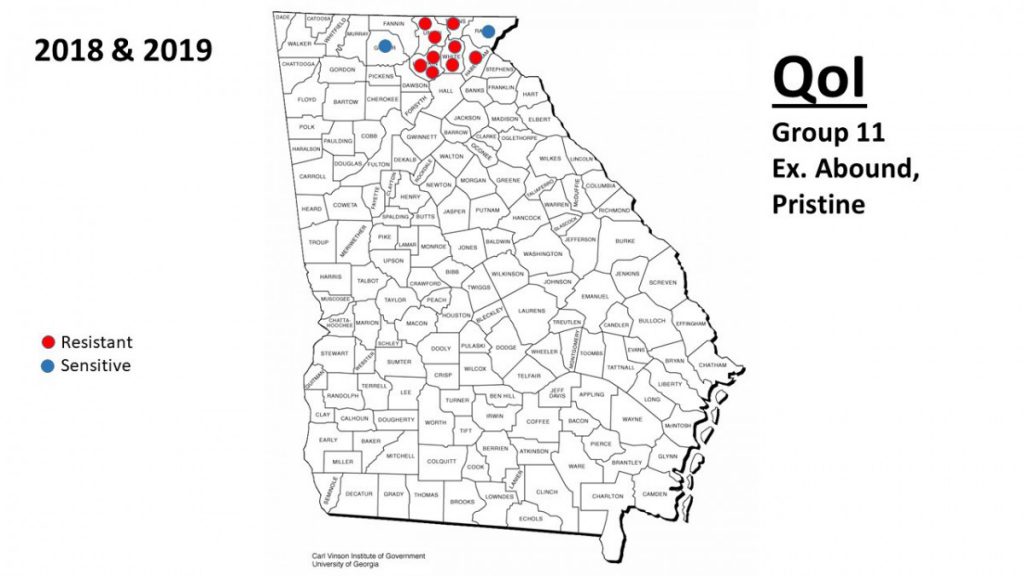
It has been previously shown that powdery mildew has the ability to develop resistance to many different classes of fungicides, but it is important to know whether this is occurring at the local level. Fungicide resistance in powdery mildew had not yet been confirmed in Georgia. Through funding provided through a USDA-SCRI grant in collaboration with Washington and several other states, surveys were initiated in 2018 and 2019 to sample vineyards with powdery mildew in order to test for QoI sensitivity or resistance. To determine whether a sample was sensitive or resistant to QoI fungicides, colonies were collected using sterile cotton swabs and sent to the USDA-ARS in Corvallis, Oregon, where the cytochrome b gene was amplified and sequenced. If the G143A mutation, associated with complete resistance to all FRAC 11 fungicides, was found, then the samples were recorded as resistant (Grasso et al., 2006). If the mutation was not found, the samples were recorded as sensitive. Results indicated QoI resistance to be prevalent in the major wine grape growing region of Georgia (Fig. 2), with the only sensitive populations being found in newer vineyards.
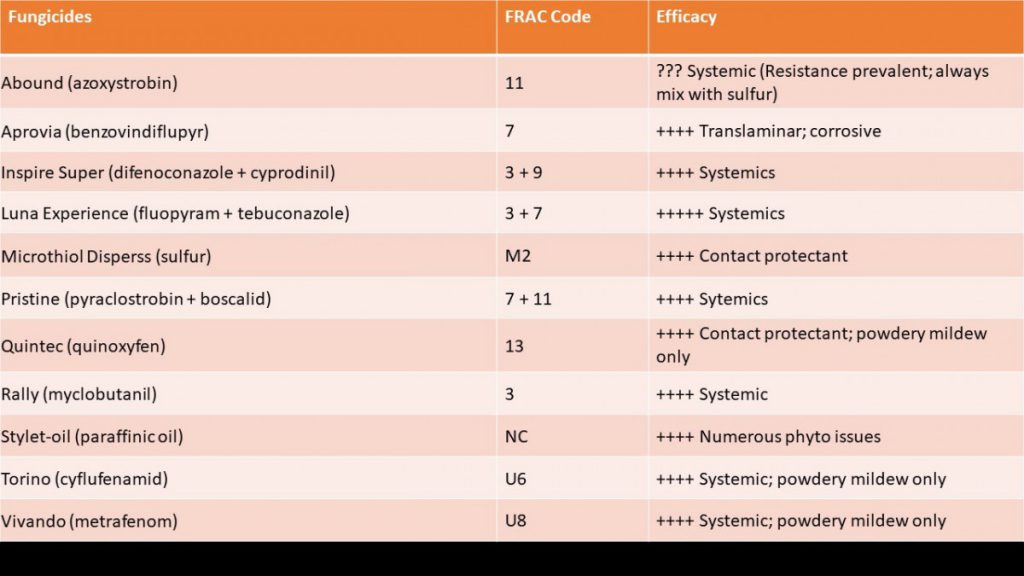
To test for potential field failures from QoIs, a field trial was conducted in 2019 at the UGA Mountain Research and Education Center in Blairsville, GA using two products containing QoIs – Abound and Pristine. In addition to these chemicals, we included various other FRAC groups with many different modes of action to see which chemicals are still efficacious against powdery mildew at this site (Table 1). As shown in Fig. 3, Abound provided no efficacy against powdery mildew. Pristine, a mixture of a QoI and an SDHI, however, still performed well. All fungicides with SDHIs (FRAC 7) provided excellent efficacy, as well as Vivando (FRAC U8) and Quintec (FRAC 13), showing there are still a variety of chemicals that can be used against powdery mildew in Georgia.
A shocking result from the field trial was the lack of control shown by Rally, a popular DMI (FRAC 3) fungicide. It has been recorded in other states that resistance has developed, but it had not been clearly shown in Georgia. To further confirm DMI resistance, samples from the research vineyard were sent to the USDA-ARS in Corvallis, Oregon to test for the Y136F mutation in the CYP51 gene associated with DMI tolerance (Délye et al., 1997). Out of seventy-five samples, all had the mutation. As shown in Fig. 3, the efficacy of Rally was only slightly better than Abound, but it was still not anywhere close to acceptable for powdery mildew management.
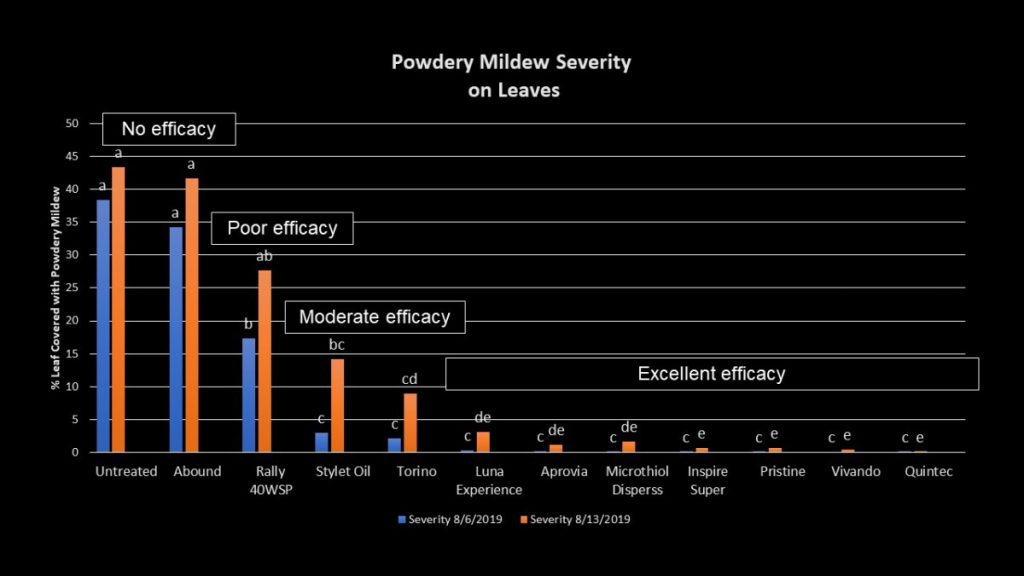
In conclusion, like downy mildew, powdery mildew has developed QoI (FRAC 11) resistance in most locations surveyed. Products like Pristine that also contain chemicals with other modes of action are still providing good efficacy, but only because of the additional SDHI chemical. In addition to being cautious of using QoIs, it is important to be knowledgeable of how many DMI (FRAC 3) fungicides are being included in a spray program. Though we do not yet know whether DMI resistance is prevalent in Georgia vineyards, the research from Blairsville shows that this resistance can be equally dangerous. It is therefore now recommended that one add sulfur with either DMI or QoI fungicides if one is unclear as to whether resistance has developed; in fact, for resistance management it is a good idea to mix sulfur at low rates with DMIs as often as possible anyway. Since QoI resistance has become widespread, it is important to keep DMIs active against powdery mildew for as long as possible through using proper resistance-management strategies. Of course, sulfur can’t be utilized with all grape varieties/species due to potential damage, but thankfully, many of the highly sulfur sensitive grapes also have limited powdery mildew issues. Without proper rotations of fungicides, other chemicals that are still providing control could develop resistance in the future, and that would leave growers with even less tools to manage powdery mildew.
Good resistance management techniques are critical as we move forward with developing and maintaining our grape spray programs. Where utilized for a number of years, the QoI (FRAC 11) fungicides are no longer active on downy mildew, powdery mildew or Botrytis, and it is likely that resistance has developed or will soon develop with other grape pathogens as well. It is possible that the QoIs are still active against some grape pathogens, but based on surveys and field research, it is difficult to see a way forward for this fungicide class in long-established vineyards. New vineyards can likely use these chemicals, especially if they are isolated from other vineyards, but their days are always numbered. The QoIs provide a warning of what can happen to new fungicide classes, as they can become useless within a relatively short period of time if overutilized. Unfortunately, the pipeline for new classes has all but dried up. For the chemistries that remain, use them wisely, or they too will go the way of the QoIs.
References
CAED. 2018. Grape farm gate value 2017. Pages 51-52 in: 2017 Georgia Farm Gate Value Report AR18-01. College of Agricultural and Environmental Sciences. University of Georgia, Athens, GA.
Grasso, V., Palermo, S., Sierotzki, H., Garibaldi, A., Gisi, U., Schwein, P., et al. 2006. Cytochrome b gene structure and consequences for resistance to Qo inhibitor fungicides in plant pathogens. Pest Manag. Sci. 472:465–472.
Délye, C., Laigret, F., and Corio-Costet, M.F. 1997. A Mutation in the 14-Demethylase Gene of Uncinula necator That Correlates with Resistance to a Sterol Biosynthesis Inhibitor. Appl. Environ. Microbiol. 63:2966-2970