By Emran Ali, Phillip M. Brannen, and Tammy Stackhouse
Fungicide resistance is a major problem for growers, as it can lead to loss of disease control, reduced yields, and unnecessary expense by applying products that no longer work. Fungal pathogens are managed with a limited number of fungicide classes. There is, therefore, a high risk of disease control failure due to potential fungicide resistance development against these fungicide classes. Pathogens can become resistant to a fungicide class through various means, with at times a single base pair mutation conferring resistance. For the proper management of fungal pathogens, early, rapid, and accurate testing methods are required to identify fungicide resistance in various fungi.
The University of Georgia Plant Molecular Diagnostic Laboratory (MDL), directed by Dr. Emran Ali, provides fungicide resistance testing support and routine advanced disease diagnosis to extension and research personnel, commercial growers, and homeowners for a wide range of plant pathogens. With financial support provided by the Southern Region Small Fruit Consortium in 2020, a total of 175 suspected strawberry samples were received from growers or extension county agents in seven southern states and tested against different fungicide classes. These states included North Carolina, South Carolina, Virginia, Tennessee, Arkansas, and Georgia.
The overall fungicide resistance frequencies were analyzed based on 145 Botrytis spp. (gray mold) isolated from six member states and 30 Colletotrichum spp. (anthracnose) isolated from three states. Results showed that the efficacy of pyraclostrobin (QoI fungicide) against both Botrytis and Colletotrichum spp. is decreasing (Figure 1). Thiophanate methyl (example product: Topsin M) is also showing weakness due to the increasing resistance phenotype against Botrytis spp. (Fig. 1).
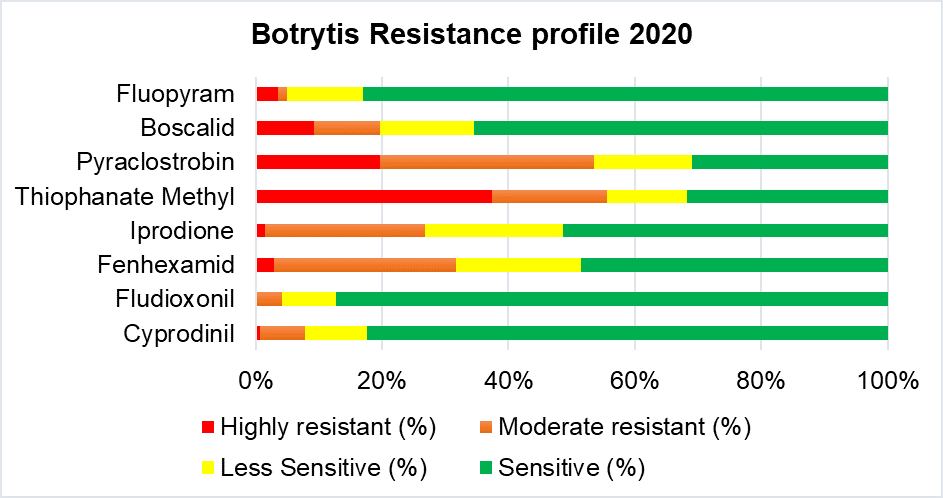
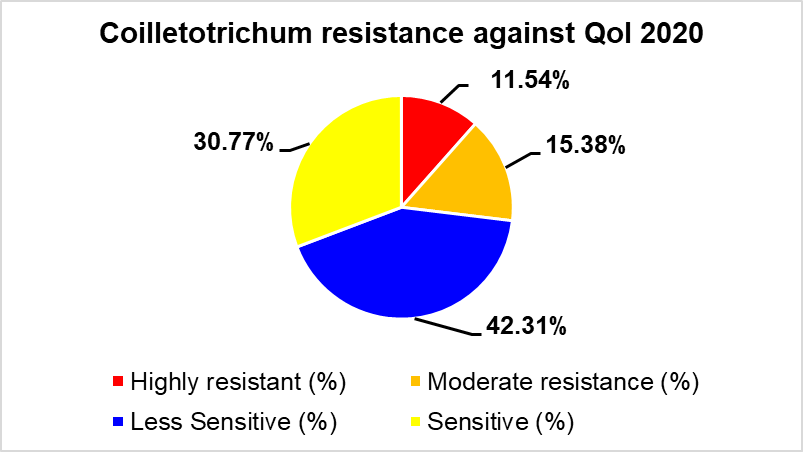
Figure 1. Fungicide resistance frequencies in 2020 for Botrytis and Colleotrichum.
This MDL program allows for a single location in the Southeast that can provide resistance testing for multiple fungal organisms and multiple fungicides. The overall goal of this program is to optimize fungicide resistance testing and to establish a system to provide support to growers and crop advisers. This program is crucial to guide growers in the use of effective fungicides to reduce losses caused by fungicide resistance. The Southern Region Small Fruit Consortium has once again provided funding for 2021 to provide this service free of charge to small fruit producers in member states – until the funds run out. After that point, producers will need to pay for the service. Continue reading for information on sample collection, submission, and processing.
Fungicide Resistance Sample Collection:
The MDL at the University of Georgia in Tifton provides fungicide resistance profiles for Botrytis cinerea and Colletotrichum spp. from strawberries and other small fruits.
For Botrytis cinerea (gray mold) samples, the MDL accepts samples from flowers, leaves, and fruit. You may send cotton swabs with spores from fruit for analysis as shown in Figure 2.

Figure 2. Appropriate and inappropriate samples for Botrytis fungicide resistance testing. Photos courtesy of Guido Schnabel at Clemson University.
- For all sample types: Place each individual flower/fruit/swab in individual sealable plastic bags. Make sure your specimens (flowers/leaves/fruits/swabs) are taken throughout the area and represent the entire field.
- For flowers and leaves: Send 20 to 40 dead strawberry flowers from each strawberry field you would like to have tested.Send as many dead leaves as you like in addition to the flowers.
- For fruits: Please send 15 to 20 symptomatic strawberry fruits (individually bagged in sealed plastic bags).
- For swab collection: Buy cotton swabs and collect spores with swabs from 15 individual berries with fresh Botrytis lesions. Use a fresh cotton swab for each berry and carefully rub one side of the swab on the diseased portion of each berry without getting strawberry juice on the swab. The swab should look lightly gray (lower right image in Fig. 2). A tiny bit of gray color is sufficient for analysis.
- Proceed to shipping section below.
For Colletotrichum (anthracnose) samples, the MDL accepts only fruit with disease symptoms (Fig. 3).

Figure 3. Appropriate sample for Colletotrichum fungicide resistance testing.
- Please send 15 to 20 freshly collected symptomatic strawberry fruits. Place each fruit in an individual sealed plastic bag. Make sure specimens are taken throughout the area and represent the entire field.
- Proceed to shipping section below.
Shipping guidelines for all fungicide resistance testing samples:
- Collect fresh samples following the guidelines in the above sections. Provide adequate amounts of each sample and ensure each sample is in an individual sealed plastic bag.
- Keep samples refrigerated after collection until they are shipped.
- Please fill the submission form, available at https://site.caes.uga.edu/alimdl/resistance-test-submission-form/, as completely as possible, including the submitter information, spraying history, host species, sampling location, pathogen being tested, etc. Incomplete forms can delay results.
- Place all specimens for a given set in a sealed plastic bag, and clearly label the outside of the bag with a permanent marker.
- If you include multiple bags in a package, clearly indicate whether each bag corresponds to a separate test or whether the bags represent multiple samples for the same test. If you have separate fields far from one another, multiple sets are recommended.
- To prevent damage during transit, ship prepared samples in cardboard boxes and add packing material such as newspaper. Do not ship prepared samples in paper bags.
- Send samples via overnight delivery to: Plant Molecular Diagnostic Lab, Plant Science Building, 2360 Rainwater Rd., Tifton, GA 31793.
Fungicide Sample Processing at the MDL:
Based on a protocol developed from Dr. Guido Schnabel at Clemson University, samples are tested using the following procedures:
- Samples from flowers, leaves, fruit, and cotton swabs with spores from fruit will be received for analysis (as shown in Fig. 2).
- Suspected samples will be incubated for several days in a moist chamber. This initial incubation process allows the pathogen to grow and sporulate on the sample surface.
- Then the pathogen will be transferred onto the centers of fungicide-amended plates and nonamended control plates.
- Microplates will be incubated for 5 days at 22°C (approximately 72°F) before measuring the radial growth in two perpendicular directions and determining the pathogen’s sensitivity to the respective fungicide.
- To better understand the fungicide resistance of a given location, several isolates (up to ten) are tested and their sensitivity compared. In general, this process takes about 7 days to make a final decision on fungicide sensitivity.
Any questions about these services including costs, mailing, and information about the other sample types we process should be directed to Emran Ali at 229-386-7230 or alimdl@uga.edu.
The Plant Molecular Diagnostic Lab has many services meant to help growers with pathogen issues. Detailed information about hosts and pathogens tested can be found at: https://site.caes.uga.edu/alimdl/.