By Jonathan E. Oliver, Department of Plant Pathology, University of Georgia
Background
Numerous cane diseases can reduce the yield and lifespan of caneberries, and the warm, humid environment in the southeastern U.S. can provide ideal conditions for disease development. Among the diseases affecting caneberry production, one of the most devastating is cane blight. This disease can rapidly spread under favorable disease development conditions, causing dieback of affected canes and ultimately the decline and death of entire plantings (Figure 1). Unfortunately, once it begins spreading and becomes established within caneberry plantings, cane blight can become challenging and/or nearly impossible to control effectively and its management poses a significant challenge to growers. Furthermore, in addition to cane blight, other cane diseases capable of causing dieback on affected canes appear to be present in southeastern caneberry plantings, and knowledge regarding the organisms causing cane dieback and appropriate management strategies remains limited.

Cane Blight Causal Agent, Infection Process, and Disease Cycle
Cane blight is caused the fungal pathogen Leptosphaeria coniothyrium (also known as Paraconiothyrium fuckelii). This pathogen infects canes via wounds, and it has been suggested that without wounds for entry, resulting disease issues would be slight (Williamson 2017). Unfortunately, in a typical caneberry planting, potential causes of wounding are abundant. Wounds resulting from cane injury via pruning, machinery, insect damage, freeze damage, herbicide damage, or infection with other pathogens can all provide entry for the cane blight pathogen (Brannen and Krewer 2005). Furthermore, canes can also self-wound (especially thorned cultivars) by rubbing one another or by rubbing on the trellis wire in windy conditions, providing additional opportunities for the pathogen to gain entry.
Once the pathogen enters through wounds, it can form lesions that spread through the vascular tissue of the plant. These spreading lesions eventually grow together and girdle the cane, resulting in death of the portion of the cane above the lesion site (Figure 2A, B). Within these lesions, on the surface of the cane, the fungus will produce small, black, embedded pimple-like structures which release fungal spores (Figure 2C). During rainfall events, these fungal spores are exuded and splashed into wounds on nearby canes, allowing for subsequent rounds of infection to occur.
Accordingly, some key events in the disease cycle for cane blight are as follows:
- In early spring, on floricanes [2nd year canes] infected during the prior season as primocanes [1st year canes], fungal structures embedded within lesions on the cane surface become evident.
- During spring and summer rain events, fungal spores are produced from these structures on floricanes.
- Fungal spores are splashed by the rain onto primocanes, where they infect through wounds.
- After infection, the fungus forms vascular lesions within infected primocanes. (These lesions may not become visible on the surface of the cane until the following season.)
- Vascular lesions spread within infected primocanes during the autumn and winter, causing the death of buds, lateral shoots, and eventual dieback of the entire cane after girdling occurs.
- The fungus overwinters within lesions on primocanes; then, the cycle begins again as in #1 above.
Cane Blight Signs and Symptoms
Visible symptoms of cane blight include lesions on primocanes and floricanes which can grow together, girdling the cane and resulting in cane death (Figure 2A). Initially, lesions may be visible near wounds as dark red areas with purple borders. Lesions eventually become gray in appearance (Figure 2B) and may be silvery due to the presence of fungal spore masses that dry on the cane surface (Williamson 2017). Within lesions, fungal spore-producing structures may be evident as black bumps (Figure 2C).

Credit: Brannen and Krewer 2005.

Credit: Ellis 2008.
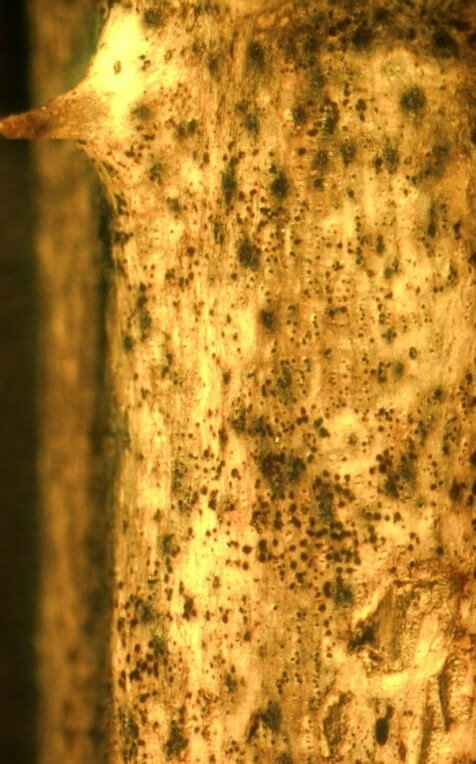
Credit: Brannen and Krewer 2005.
Other Potential Causes of Cane Dieback
While cane blight is assumed to be the primary cause of cane dieback in the southeastern U.S., recent evidence suggests that other fungal organisms may also be causing cane dieback of blackberry. During the 2017, 2018, and 2019 growing seasons, fungal organisms were isolated from blackberry plants showing symptoms of cane dieback (Figure 3A) in eight counties in Georgia (Hemphill et al. 2020). Isolates were identified and used to inoculate cut (wounded) cane terminals on potted blackberry plants to determine if they were capable of causing cane dieback. Among the 126 isolates identified in this work, the causal organism of cane blight, L. coniothyrium, was identified from only one location on plants showing cane dieback symptoms, while isolates of Fusarium oxysporum, Pestalotiopsis microspora, Colletotrichum siamense, Neofusicoccum kwambonambiense, N.parvum, Lasiodiplodia pseudotheobromae, and L. theobromae were determined to cause significant dieback on wounded blackberry canes (Figure 3B, C). Taken together, this information suggests that additional fungal organisms besides L. coniothyrium are present and contributing to cane dieback in the southeastern U.S. Furthermore, as multiple fungal isolates capable of causing significant cane dieback could sometimes be isolated from a single diseased cane in this study, it is likely that a disease complex may be involved in the observed field symptoms.



Management
Due to the significance of wound infections and rainfall in the cane blight disease process, recommendations for cane blight management focus heavily on the prevention of unnecessary wounding and the protection of wound sites prior to rainfall events via fungicide applications. No information is available for blackberry regarding cultural controls for other cane dieback-causing organisms; however, based on the lifestyle of these organisms, it is reasonable to assume that cultural management practices recommended for cane blight would likely also be effective for the other causes of cane dieback.
Cultural Controls for Cane Blight and Cane Dieback
Cultural control recommendations for cane blight and cane dieback management include the following:
- Minimize wounding of primocanes. Wounds provide an opening for cane blight and cane dieback organisms to gain entry into the plant and an opportunity for infection. Pruning activities will inevitably lead to wounding, but these activities are necessary in caneberry production operations. Since fungal spores can be spread through rainfall (or overhead irrigation) events, care should be taken to avoid pruning prior to these events. Ideally, pruning should take place when at least four days of dry weather are expected.
- Whenever possible, “pinch off” or “tip” tender primocanes rather than relying on severe pruning cuts with shears. “Tipping” primocanes when they reach the desired height (and continuing to “pinch”-prune during summer pruning) will result in minimal damage to the cane and will allow for quick healing of wounds. To use this method, pruning of primocanes must be timed appropriately, since it will become necessary to use pruning shears once canes become too tall. [For additional information on “pinching” and “tipping”, see the UGA publication “Cane Blight of Blackberry” available at https://extension.uga.edu/publications/detail.html?number=C894.]
- After harvest, promptly remove infected canes and old floricanes. Since infected canes and old floricanes serve as a reservoir for fungal organisms that can cause future infections, these canes should be pruned out and immediately destroyed (by burning or burying) after harvest. Pruning cuts should be made close to the ground, since remaining stumps can harbor fungal organisms.
- Implement practices that promote quick drying of the canopy. These include thinning plants, establishing a weed-free strip, bedding with black plastic, and using drip (rather than overhead) irrigation. Keeping the canopy dry will decrease fungal infection.
- Maintain adequate water and nutrient conditions to avoid stressing plants. Stressed plants are more susceptible to infection with fungal organisms, and wounds are likely to heal more quickly in healthy plants. Use soil and tissue sampling to ensure adequate fertilization and pH.
Chemical Controls for Cane Blight and Cane Dieback
Chemical control recommendations for cane blight and cane dieback management include the following:
- Protect wound sites by applying fungicides after each day of pruning. This can help to protect wound sites from fungal infection until healing can occur.
- For cane blight: apply effective fungicides. Strobilurin fungicides (FRAC Group 11), such as including Pristine, Cabrio, Abound, and Quilt Xcel, and the DMI fungicide Rally (FRAC Group 3 have all shown efficacy when applied to pruning wounds to protect canes from cane blight.
- For cane dieback causing organisms: apply effective fungicides. Information is very limited regarding chemical controls for cane dieback. However, a recent fungicide trial on potted blackberry plants indicated that Pristine, Switch, and (to a lesser extent) Abound can reduce cane dieback from wounds if these materials are applied prior to infection by select fungal organisms identified previously to cause cane dieback in Georgia (Oliver et al. 2020).
For additional disease control recommendations for blackberry production, please see the Southeast Regional Caneberry Integrated Management Guide (at www.smallfruits.org). Fungicide availability, labels, and recommended rates change frequently and vary between states and localities. Please consult the various labels for rates, other recommendations, and precautions.
References
Brannen, P. and Krewer, G. 2005. Cane blight of blackberry. UGA Circular 894. https://extension.uga.edu/publications/detail.html?number=C894
Ellis, M. A. 2008. Cane blight of raspberries. Ohio State University Extension. https://ohioline.osu.edu/factsheet/plpath-fru-10
Oliver, J. E., Hemphill, W. H., and Brannen, P. 2020. Evaluation of fungicides for reducing cane dieback on blackberry canes after pruning in Georgia, 2019. PDMR 14:PF032.
Hemphill, W., Brannen, P. M., and Oliver, J. E. 2020. Identification of organisms associated with cane dieback of cultivated blackberry (Rubus fructicosis) in Georgia. Phytopatholgy 110(12S):S2.115-S2.116.
Williamson, B. 2017. Cane Blight pg. 11-13 in Compendium of Raspberry and Blackberry Diseases and Pests, 2nd Edition. Martin, R., Ellis, M., Williamson, B. and Williams, R., eds. APS, St. Paul, MN.